Alv B has signed a licensing agreement with Diakonos for exclusive rights of Tumor Prep in the European market, with rights of first refusal for the global market.
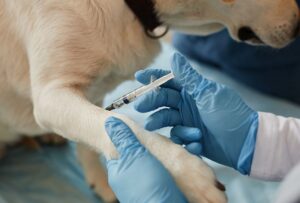
Tumor Prep is a six-ingredient off-the-shelf cancer treatment that is administered locally in three weekly doses. The six compounds combine three mechanisms of action to target tumors and eradicate cancer cells. These mechanisms target processes essential to cancer survival, including intracellular pattern recognition, TGF-β inhibition, and cytostasis/cytoreduction. The treatment has been tested in multiple model systems, where it has consistently cured up to 50% of animals while substantially reducing growth and metastasis in all animals that have received Tumor Prep. Importantly, Tumor Prep has already been shown to be an effective treatment for cancer in dogs. We are extremely excited to report that multiple dogs with mammary cancer treated with Tumor Prep saw reduction in tumor sizes and many to the extent they have now been cured post-surgery.
Alv B plans to continue this excellent research in Europe to further show the efficacy of Tumor Prep against cancer in dogs. Working together with our already establish veterinary partners, we plan to set up a new clinical trial in Norway with the goal to bring this revolutionary cocktail to market. Through the regulatory process for marketing authorization in Europe, we aim that Tumor Prep can be available for all.
Pending formal acceptance from Baylor College, we have signed an agreement of the license, entitled: “Potentiation of durable antitumor immunity by multifactorial immune modulation”. The agreement allows Alv B exclusive rights to Europe and right to the first refusal for the global market. This represents an exciting and important move by Alv B, in our mission to bring better cancer care for dogs across Norway and the world.